TruTechnologies' sample tracking platform prevented unconsented PK samples from being collected, which allowed a prediatric patient to go home hours early.
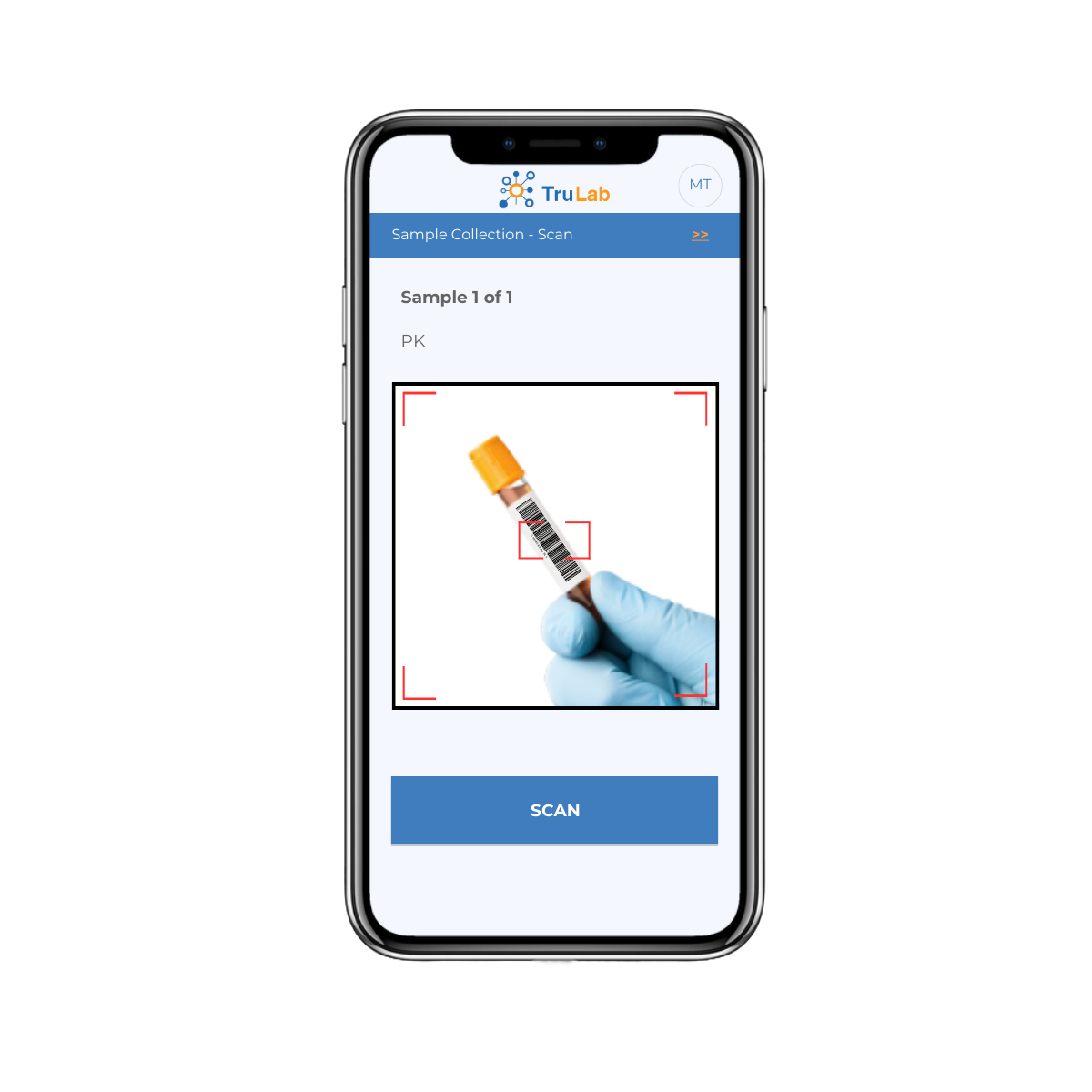
In the course of alerting the site, TruLab surfaced unnecessary 4-hour and 8-hour PK samples the site was about to collect. As a result, a young boy not only avoided extra blood draws but also went home much earlier than expected.
In the course of alerting the site, TruLab surfaced unnecessary 4-hour and 8-hour PK samples the site was about to collect. As a result, a young boy not only avoided extra blood draws but also went home much earlier than expected.
To ease patient burden, a recent protocol amendment eliminated the 4-hour and 8-hour PK for a visit later in the enrollment period.Clinical sites can sometimes overlook subsequent amendments and conduct visits according to previous versions of the protocol, including material changes to the schedule of assessments.
Being a rare disease study, they recognized that maintaining a direct communication line with the sites enrolling patients would be critical to optimizing enrollment. TruTechnologies, positioned at the forefront of site operations and trusted by clinical research coordinators, was seen as the ideal solution to bridge this gap.
The sponsor requested a configuration that would not only enable source data capture at the point of sample collection but also facilitate the logging of screening activities from the outset — before the first sample was even collected. Through a combination of strategic actions, including close collaboration with sites and timelyvisibility into recruitment activities via TruLab, the sponsor recognized a 10-month improvement in timeline projections compared to prior studies.
Based on prior experience, the sponsor’s head of clinical pharmacology was already an advocate for TruLab’s capabilities in advanced sample tracking and protocol oversight, particularly given the importance of a circulating biomarker in this study.Delays stemming from collection of these logistically sensitive samples are common, often resulting from missing data, mishandling, or prolonged transit times that risk their stability. When these samples become unusable, prompt redraws are essential.
However, past trials highlighted an even more pressing bottleneck: the screening and enrollment phase at the site level. Even with a CRO’s assistance in coordinating these activities, the sponsor often felt left in the dark, lacking critical information about site progress. This communication gap not only made enrollment fore casting difficult but also limited the sponsor's ability to proactively support sites in this demanding rare disease study. It’s challenging for the sponsor to be on standby for multiple sites around the world without insight into when or where patients are being screened.
.png)
On this particular visit, a little boy went to the clinical site and was set for a long day with an IV in his arm followed by hours of blood tests.Prior to the amendment, the schedule called for blood draws ofPK samples that ended 8 hours after the infusion.
As the boy began his visit,TruLab detected an anomaly. AsTruLab spoke with the site, they discovered this site missed therecent protocol amendment which eliminated the last two blood draws at the 4-hour and 8-hour timepoints, and they were intending on collecting those samples.
TruLab informed the site of the amendment, making for an easier day for the boy and turning a potentially 10-hour visit into 3 hours. Because TruTechnologies' platform and monitoring services prevented this deviation, instead of spending all day at the clinical site with unnecessary blood draws, the little boy got to go home early.
•The young boy avoided unnecessary samples and time at the site
•TruTechnologies notified the site of the most recent protocol amendment
•TruTechnologies prevented a protocol deviation before it happened
Conclusion
Sites can easily misinterpret protocols or miss subsequent protocol changes amid everything else happening at a site. TruLab ensures everything complies with the latest protocol amendments, guiding sites through the correct collections. The TruTechnologies monitoring team not only catches deviations as they occur but also prevents the mix-ups from occurring in the first place.
